Homemade Chemistry Set
These experiments are all ones I have found suitable for younger experimenters. They are easy to set up, easy to clean up after, and do not use any chemicals not found in the kitchen. You still need to be careful and follow lab safety procedures, and provide adult supervision and explanation.
Weak Acids
Household white vinegar is basically Acetic acid and water. Usually vinegar is 3-5 percent acetic acid, with the strongest kitchen vinegars capping out at 10 percent. Distillation and fractional freezing can be used to increase that concentration, but only up to a point (maybe 25-30 percent). Still, vinegar is a useful acid for home chemistry experiments with a weak acid.

Dissolving eggshells in vinegar #1
Eggshells are mostly calcium corbonate. As a result if you soak egg shells in acetic acid (vineagar and water) the calcium carbonate disollves the eggshells. The reaciton generates CO2 gas.
CaCO3 (s) + 2 HC2H3O2 (aq) -> Ca(C2H3O2)2 (aq) + H2O (l) + CO2 (g)
By redirecting the off gassed CO2 into a fluid filled filled test tube you get a sense for just how much gass is being generated. Another way is with the balloon filling eperiment.

Dissolving eggshells in vinegar #2
The chemistry is the same as our previous eggshell and vinegar demo. The vinegar still reacts with the calcium carbonate in the eggshells to generate CO2 gas, but in this demo we capture the CO2 in a balloon. The balloon gives another way to visualize volume and rate of CO2 gas production.

Dissolving the eggshell off an egg
The vinegar is able to dissolve of the shell material, without damaging the inner membranes under the shell. Turns out you can do a bunch of interesting things with an egg if you first dissolve off the shell first; generally they are then called naked eggs.
Since the inner two membranes of the egg are semi-permeable you can do a number of osmosis experiments showing materials being added to, or removed from, the egg by osmosis.
Sublimation Experiments
Sublimation describes a phase change where a solid turns directly into a gas without passing through a liquid state.
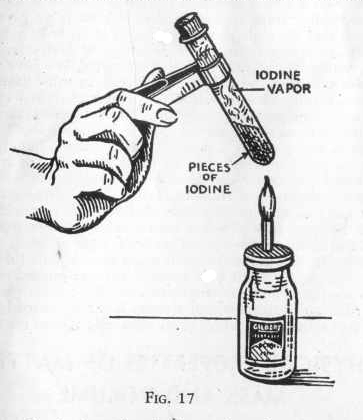
Iodine Sublimation
Iodine sublimates at very low temperatures and creates a very visible purple gas. As a result heating iodine, and watching it sublimate is one of the classic chemistry demonstrations. It is also quite beautiful.